The lewis structure is a demonstration of valence shell electrons in a molecule. In the case of IF5 The Iodine atom has 7 valence electrons.

Lewis Diagrams Made Easy How To Draw Lewis Dot Structures Youtube
Steps of drawing NO 3-lewis structure.

. A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Let us consider the Lewis structure of benzene step by step-Step 1 The first step is the determination of the total no. Then after completing octets for all the atoms that need them place remaining electrons on the central atom IF the central atom is a Period 3 or greater element.
Count total valence electron in SeF4. F also has 7. Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom.
In lewis diagrams valence electrons are represented by dots. For more help watch this general tutorial on drawing Lewis Structures for compounds like the Sulfur trixoide molecule. First of all determine the valence electron that is available for drawing the lewis structure of SeF4 because the lewis diagram is all about the representation of valence electrons on atoms.
Now we move on to the steps of drawing a Lewis diagram-1. It is helpful if you. It shows how individual electrons are arranged in a molecule.
Lewis Structure of nitrite ion. So when we draw the Lewis diagram for the Cl atom we draw 7 dots around it. Now we are going to learn how to draw this lewis structure of NO 3-ion.
For the PH3 Lewis structure we first count the valence electrons for the PH3 molecule using the periodic table. Drawing the Lewis Structure for PH 3. Drawing the Lewis Structure for PH 3.
Find total number of electrons of the valance shells of nitrogen and oxygen atoms and including charge of the anion. Try to draw the SO 3 Lewis structure before watching the video. Watch the video and see if you missed any steps or information.
Follow some steps for drawing the lewis dot structure of SeF4. The first step is to count all the valence electrons of each molecule. Drawing the Lewis Structure for SO 3.
8 After you have completed your Lewis structure CHECK FOR OCTETS. Shared pairs of electrons are drawn as lines between atoms while lone pairs of electrons are drawn as dots next to atoms. So an easy way to find the valence electron of atoms in the SeF4 molecule is just to look at.
When constructing a Lewis diagram keep in mind the octet rule which refers to the tendency of atoms to gain lose or share electrons until they are. If all atoms have an octet calculate formal charges for. Available steps 2-4 above.
It is also known as an electron dot structure where each bond is shown as two dots between two atoms. Drawing Lewis Structures A step-by-step Guide by C. Following steps are required to draw NO 3-lewis structure and they are explained in detail in this tutorial.
Be sure to check the formal charges for the Lewis structure for SO 3.
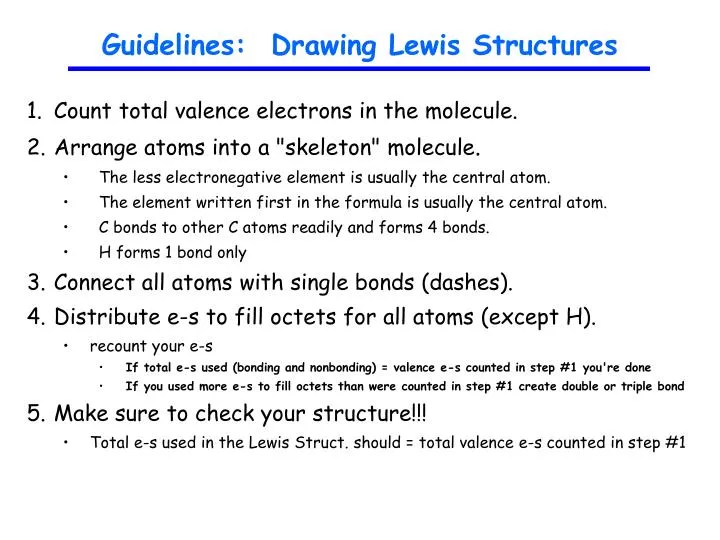
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Chemistry 101 Draw Lewis Dot Structures For Covalent Compounds Youtube

How To Draw Lewis Structures A Step By Step Tutorial Middle School Science Blog

Lewis Structures 5 Steps 1 Count Valence E Available If An Anion Add Charge To Valence E If A Cation Subtract Charge From Valence E 2 Draw Skeleton Ppt Download

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Youtube
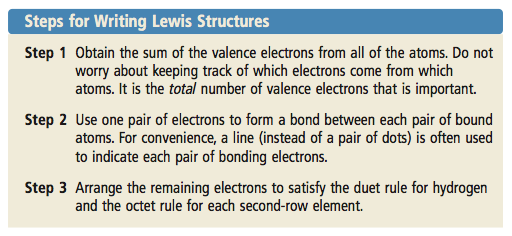

0 comments
Post a Comment